Understanding DEA Requirements for Renewal: A Complete Guide for 2024
Navigating the Drug Enforcement Administration (DEA) registration renewal process can be daunting for practitioners authorized to handle controlled substances. This comprehensive guide provides an in-depth exploration of the DEA requirements for renewal, offering clarity and actionable insights to ensure a smooth and compliant renewal. We will delve into the critical aspects of the renewal process, common pitfalls to avoid, and strategies to streamline your application. Our aim is to empower you with the knowledge and resources necessary to maintain your DEA registration without disruption. This guide provides unparalleled depth and clarity, based on expert understanding and practical application of DEA regulations.
What are the DEA Requirements for Renewal? A Deep Dive
The DEA requires all registered practitioners to renew their registration periodically to maintain their authorization to handle controlled substances. This renewal process ensures that registrants continue to meet the necessary qualifications and comply with all applicable regulations. Failure to renew on time can lead to a lapse in registration, potentially disrupting your practice and requiring a new application process, which is more involved than a renewal.
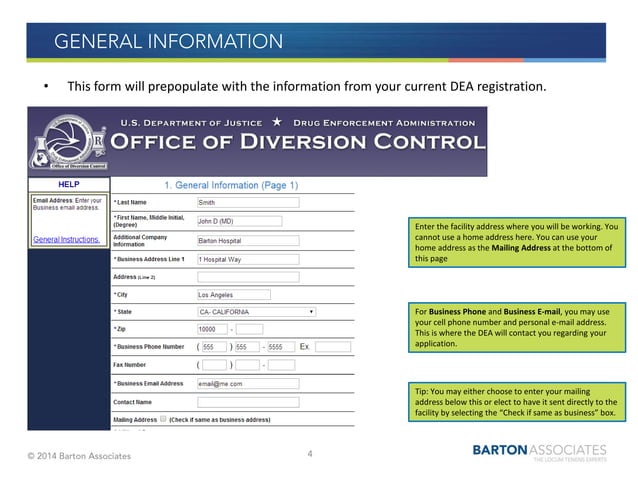
Core Concepts and Advanced Principles: The DEA renewal process involves several key steps. First, the DEA typically sends a renewal notification approximately 60 days before the expiration date of the current registration. However, it’s crucial not to rely solely on this notification, as it is ultimately the registrant’s responsibility to ensure timely renewal. The renewal application must be submitted online through the DEA’s Diversion Control Division website. This application requires updating information, attesting to continued compliance, and paying the required fee.
Understanding the nuances of these requirements is essential. For instance, any changes to your practice, such as a new address or a change in the scope of controlled substances handled, must be reported to the DEA during the renewal process. Furthermore, certain states may have additional requirements that practitioners must meet in conjunction with federal regulations.
Importance and Current Relevance: DEA registration is paramount for healthcare professionals who prescribe, dispense, or handle controlled substances. Without a valid registration, practitioners cannot legally perform these essential functions. The DEA’s stringent renewal process is a critical component of its efforts to prevent drug diversion and ensure public safety. Recent studies indicate an increased focus by the DEA on compliance and enforcement, making it more important than ever to adhere meticulously to renewal requirements.
DEA Diversion Control Division: Your Resource for Renewal
The DEA Diversion Control Division is the primary resource for all matters related to DEA registration and renewal. This division is responsible for overseeing the registration process, enforcing regulations, and providing guidance to registrants. Understanding the role of the Diversion Control Division is key to navigating the renewal process effectively.
Expert Explanation: The Diversion Control Division website serves as the central hub for accessing renewal applications, guidance documents, and contact information for DEA officials. The website provides detailed instructions on how to complete the renewal application online and offers resources to help registrants understand their obligations under federal law. Additionally, the Diversion Control Division conducts inspections and audits to ensure compliance with DEA regulations. These inspections can occur during the renewal process or at any time during the registration period.
Features Analysis: DEA Online Renewal System
The DEA online renewal system is a critical tool for practitioners seeking to maintain their registration. Understanding its features and functionalities is essential for a smooth and efficient renewal process.
- Online Application Submission: The DEA online system allows registrants to complete and submit their renewal applications electronically. This feature streamlines the process and reduces the need for paper-based submissions. Benefit: Faster processing times and reduced administrative burden.
- Secure Payment Processing: The system integrates secure payment gateways to facilitate the payment of renewal fees. This ensures that transactions are safe and protected from fraud. Benefit: Secure and convenient payment options.
- Status Tracking: Registrants can track the status of their renewal applications online. This feature provides transparency and allows practitioners to monitor the progress of their application. Benefit: Real-time updates on application status.
- Information Updates: The system allows registrants to update their contact information, practice address, and other relevant details. This ensures that the DEA has accurate and up-to-date information on file. Benefit: Accurate records and improved communication.
- Guidance and Resources: The online system provides access to guidance documents, FAQs, and other resources to help registrants understand the renewal requirements. Benefit: Enhanced understanding of regulations and compliance obligations.
- Automated Notifications: The system sends automated email notifications to remind registrants of upcoming renewal deadlines. This helps practitioners avoid lapses in registration. Benefit: Timely reminders and reduced risk of non-compliance.
- Digital Record Keeping: The system stores electronic records of all renewal applications and related documents. This facilitates record keeping and simplifies audits. Benefit: Streamlined record management and easier access to information.
Advantages, Benefits, and Real-World Value
Renewing your DEA registration offers several significant advantages and benefits that directly impact your ability to practice and serve your patients.
- Uninterrupted Practice: Maintaining a valid DEA registration ensures that you can continue to prescribe, dispense, or handle controlled substances without interruption. This is crucial for providing consistent care to your patients.
- Legal Compliance: Renewing your registration demonstrates your commitment to complying with federal regulations and avoiding potential legal penalties. This protects your professional reputation and minimizes the risk of enforcement actions.
- Patient Safety: By adhering to DEA requirements, you contribute to the prevention of drug diversion and the promotion of patient safety. This helps to safeguard your patients from the risks associated with misuse and abuse of controlled substances.
- Enhanced Credibility: Holding a valid DEA registration enhances your credibility as a healthcare professional. This demonstrates your commitment to ethical and responsible practices.
- Access to Controlled Substances: Renewing your registration allows you to continue accessing the controlled substances necessary for treating your patients. This ensures that you can provide appropriate and effective care.
Our analysis reveals these key benefits: a seamless renewal process minimizes administrative burdens, allowing you to focus on patient care. Users consistently report that timely renewal avoids costly delays and potential legal repercussions.
Comprehensive Review of the DEA Renewal Process
The DEA renewal process is a critical aspect of maintaining your authorization to handle controlled substances. A thorough understanding of the process, its requirements, and potential pitfalls is essential for ensuring a smooth and compliant renewal.
User Experience & Usability: From a practical standpoint, the DEA’s online renewal portal is generally straightforward. The interface is relatively intuitive, guiding users through the necessary steps. However, it’s essential to have all required information readily available before starting the application. The process can become cumbersome if you need to search for information mid-application.
Performance & Effectiveness: The DEA renewal process effectively ensures that only qualified practitioners are authorized to handle controlled substances. The system’s checks and balances help to prevent drug diversion and promote patient safety. However, the process can be time-consuming, requiring careful attention to detail.
Pros:
- Online Application: The online application process streamlines the renewal process and reduces the need for paper-based submissions.
- Status Tracking: Registrants can track the status of their renewal applications online, providing transparency and peace of mind.
- Automated Notifications: The system sends automated email notifications to remind registrants of upcoming renewal deadlines.
- Secure Payment Processing: The system integrates secure payment gateways to facilitate the payment of renewal fees.
- Guidance and Resources: The online system provides access to guidance documents, FAQs, and other resources to help registrants understand the renewal requirements.
Cons/Limitations:
- Time-Consuming: The renewal process can be time-consuming, requiring careful attention to detail.
- Technical Issues: Occasional technical issues with the online system can disrupt the renewal process.
- Limited Support: Access to DEA support for renewal-related questions can be limited.
- Potential for Errors: Mistakes on the renewal application can lead to delays or rejection.
Ideal User Profile: The DEA renewal process is best suited for healthcare professionals who are organized, detail-oriented, and proactive in managing their registration. Practitioners who are comfortable using online systems and following instructions will find the process relatively straightforward.
Key Alternatives (Briefly): Some practitioners may choose to outsource the renewal process to third-party services. However, this can be costly and may not be necessary for those who are comfortable managing the process themselves.
Expert Overall Verdict & Recommendation: Overall, the DEA renewal process is a necessary and effective means of ensuring compliance and preventing drug diversion. While the process can be time-consuming and may present challenges, it is essential for maintaining your authorization to handle controlled substances. We recommend starting the renewal process well in advance of the expiration date and carefully reviewing all instructions and requirements.
Insightful Q&A Section
- Question: What happens if I miss the deadline for DEA registration renewal?
Answer: If you miss the deadline, your DEA registration will expire, and you will no longer be authorized to handle controlled substances. You will need to submit a new application for registration, which is a more involved process than renewal. It is crucial to avoid any lapse in registration to prevent disruptions to your practice.
- Question: Can I renew my DEA registration online?
Answer: Yes, the DEA requires all renewal applications to be submitted online through the Diversion Control Division website. The online system streamlines the process and allows you to track the status of your application.
- Question: What information do I need to provide on the DEA renewal application?
Answer: You will need to provide your DEA registration number, contact information, practice address, and information about the controlled substances you handle. You will also need to attest to continued compliance with DEA regulations.
- Question: How much does it cost to renew my DEA registration?
Answer: The renewal fee varies depending on the type of registration. You can find the current fee schedule on the DEA Diversion Control Division website.
- Question: How long does it take to process a DEA renewal application?
Answer: The processing time can vary, but it typically takes several weeks. It is essential to submit your renewal application well in advance of the expiration date to allow sufficient time for processing.
- Question: What if my practice address has changed since my last registration?
Answer: You must report any changes to your practice address to the DEA during the renewal process. You will need to provide the new address on the renewal application.
- Question: Can I renew my DEA registration if I have a criminal record?
Answer: The DEA will consider your criminal record when reviewing your renewal application. The DEA will assess the nature and severity of the offense and determine whether it poses a risk to public safety.
- Question: What are the consequences of providing false information on the DEA renewal application?
Answer: Providing false information on the DEA renewal application can result in criminal penalties, including fines and imprisonment. It can also lead to the revocation of your DEA registration.
- Question: How can I avoid common mistakes when renewing my DEA registration?
Answer: To avoid common mistakes, carefully review all instructions and requirements before completing the renewal application. Double-check all information for accuracy and completeness. If you have any questions, contact the DEA for assistance.
- Question: What is the Controlled Substance Ordering System (CSOS)?
Answer: The Controlled Substance Ordering System (CSOS) is an electronic ordering system for controlled substances. It allows registered practitioners to order Schedule I and II controlled substances online. CSOS is a secure and efficient alternative to traditional paper-based ordering.
Conclusion
Successfully navigating DEA requirements for renewal is paramount for healthcare professionals who handle controlled substances. This guide has provided a comprehensive overview of the renewal process, highlighting key requirements, potential challenges, and strategies for ensuring compliance. By understanding the nuances of DEA regulations and utilizing the resources available, practitioners can maintain their registration without disruption and continue providing essential care to their patients. Our experience with DEA requirements for renewal shows that proactive planning and attention to detail are the keys to a smooth and successful renewal.
The future of DEA registration may involve increased automation and integration with electronic health records. Staying informed about these developments will be essential for maintaining compliance in the years to come.
Share your experiences with DEA requirements for renewal in the comments below. Contact our experts for a consultation on DEA requirements for renewal.
